Ukuphepha kwamabhethri e-lithium namabhethri e-lead-acid bekulokhu kuyimpikiswano phakathi kwabasebenzisi.Abanye abantu bathi amabhethri e-lithium aphephile kunamabhethri e-lead-acid, kodwa abanye bacabanga okuphambene.Ngokombono wesakhiwo sebhethri, amaphakethe ebhethri le-lithium amanje ngokuyisisekelo angamabhethri angu-18650 okupakishwa, futhi amabhethri e-lead-asidi ngokuyisisekelo amabhethri e-lead-asidi angalondolozi anomsebenzi omuhle wokuvala, futhi izici zobungozi zazo zombili ziyafana ngokuyisisekelo.Ubani ophephe kakhudlwana, vele ubheke phansi futhi uzokwazi!

ibhethri ye-lithium:
Amabhethri e-lithium awuhlobo lwamabhethri asebenzisa insimbi ye-lithium noma i-lithium alloy njengento engalungile ye-electrode futhi asebenzisa isixazululo se-electrolyte esinganamanzi.Amabhethri e-lithium angahlukaniswa cishe abe izigaba ezimbili: amabhethri ensimbi ye-lithium namabhethri e-lithium-ion.Ngo-1912, amabhethri ensimbi e-lithium aqale ahlongozwa futhi afundwa nguGilbert N. Lewis.Ngenxa yamakhemikhali asebenzayo ensimbi ye-lithium, ukucubungula, ukugcinwa, kanye nokusetshenziswa kwensimbi ye-lithium kunezidingo eziphakeme kakhulu zemvelo.Ngakho-ke,amabhethri e-lithiumengakaze isetshenziswe isikhathi eside.Ngokuthuthuka kwesayensi nobuchwepheshe, amabhethri e-lithium manje asejwayelekile.
Amabhethri e-lead-acid:
Ibhethri le-lead-acid (VRLA) ibhethri lesitoreji ama-electrode awo enziwe ngokuyinhloko ngomthofu nama-oxides awo, futhi i-electrolyte yawo iyisisombululo se-sulfuric acid.Esimweni sokukhishwa kwebhethri le-lead-acid, ingxenye eyinhloko ye-electrode enhle i-lead dioxide, futhi ingxenye eyinhloko ye-electrode engalungile ingumthofu;esimweni esikhokhisiwe, izingxenye eziyinhloko zama-electrode amahle futhi angalungile yi-lead sulfate.
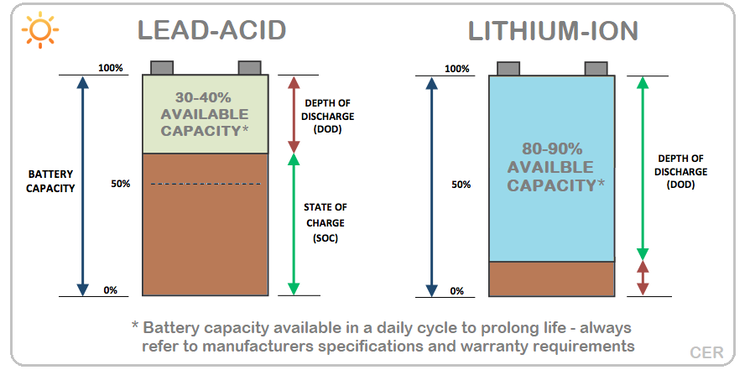
Amandla kagesi okuzisholo webhethri le-asidi yeseli elilodwa elingu-2.0V, elingashajwa liye ku-1.5V futhi lingashajwa libe ngu-2.4V.Kuzinhlelo zokusebenza, amabhethri e-lead-acid angu-6 yeseli elilodwa avamise ukusetshenziswa ochungechungeni ukuze akhe ibhethri le-lead-acid engu-12V.Kukhona futhi 24V, 36V, 48V nokunye.
Iyiphi ephephile, ibhethri ye-lithium noma ibhethri le-lead-acid?
Ngokombono wokuvikela ukuphepha kwebhethri, ama-valve okuphepha aklanyelwe kumaseli angu-18650, angakwazi nje ukukhulula ukucindezela okukhulu kwangaphakathi, kodwa futhi anqamule ibhethri kumjikelezo wangaphandle, okulingana nokuhlukanisa iseli ngokomzimba ukuze kuqinisekiswe ukuthi ukuphepha. kwamanye amaseli ebhethri ephaketheni lebhethri.Ukwengeza, amaphakethe ebhethri e-lithium ngokuvamile afakwe amabhodi okuvikela e-BMS, angakwazi ukulawula ngokunembile isimo seseli ngayinye ephaketheni lebhethri, futhi axazulule ngokuqondile inkinga yokukhokhiswa ngokweqile kanye nokukhishwa ngokweqile okuvela kumsuka wezimpande.
Ibhethri ye-Lithium uhlelo lokuphatha ibhethri ye-BMS inganikeza ukuvikeleka okugcwele kwebhethri, imisebenzi ihlanganisa: ukushaja / ukukhipha ukuvikelwa kokushisa okuphezulu nokuphansi;ukuvikelwa kwe-voltage ye-overcharge eyodwa / ukukhishwa kwe-overdischarge;ukushaja / ukukhipha ukuvikelwa kwe-overcurrent;ibhalansi yeseli;ukuvikelwa kwesifunda esifushane;Izikhumbuzi nokuningi.
I-electrolyte yeiphakethe lebhethri le-lithiumiyisisombululo esixubile sikasawoti we-lithium kanye ne-organic solvent, lapho usawoti we-lithium othengiswayo i-lithium hexafluorophosphate.Le nto ijwayele ukubola emazingeni okushisa aphezulu futhi ibhekana nokusabela kwe-thermochemical ngamanani amancane wamanzi nezinyibilikisi eziphilayo ukuze kuncishiswe ukuzinza kwe-Thermal ye-electrolyte.
Ibhethri ye-lithium yamandla ikakhulukazi isebenzisa i-lithium iron phosphate.Ibhondi ye-PO ku-lithium iron phosphate crystal izinzile futhi kunzima ukubola.Ngisho nasezingeni eliphezulu lokushisa noma ukushajwa ngokweqile, ngeke liwe futhi likhiqize ukushisa noma lenze izinto ezinamandla ze-oxidizing njenge-lithium cobaltate.Ukuvikeleka okuhle.Kubikwa ukuthi ekusebenzeni kwangempela, inani elincane lamasampuli atholakale evutha ngesikhathi sokuhlolwa kwe-acupuncture noma i-short-circuit, kodwa akukho sigameko sokuqhuma esenzeka.Ukuphepha kwamaphakethe ebhethri ye-lithium kuye kwathuthukiswa kakhulu.
Ngokuphambene, amabhethri e-lead-acid awanaso isivikelo sesistimu ye-BMS.Amabhethri aneasidi yomthofu abonakala entula ekuvikeleni ukuphepha ngaphandle kwamavalvu okuphepha.Ukuvikelwa kwe-BMS cishe akukho.Amashaja amaningi aphansi awakwazi ngisho nokucisha ngemva kokushajwa ngokugcwele.Ukuvikela ukuphepha kukude namabhethri e-lithium.Ihambisana neshaja yekhwalithi ephansi, kuhle ukuthi ube sesimweni esihle.
Ukuqhuma okuzenzakalelayo ezimotweni zikagesi kuvame ukwenzeka, iningi lazo elibangelwa ukushajwa kwebhethri nokushajwa.Abanye ochwepheshe baye bachaza ukuthi amabhethri e-lead-acid athatha isikhathi eside kakhulu ukushajwa, futhi lapho eshajwa kuze kube sekugcineni, ngemva kokuba izigxobo ezimbili ziguqulwe zibe izinto ezisebenzayo, uma eqhubeka nokushaja, kuzokhiqizwa inani elikhulu likagesi.I-Hydrogen, i-oxygen gas.Uma ukugcwala kwale gesi exubile kubalelwa ku-4% emoyeni, kuphuze kakhulu ukubaleka.Uma imbobo yokukhipha umoya ivinjiwe noma kunegesi eningi, izoqhuma lapho ihlangana nelangabi elivulekile.Izolimaza ibhethri ekukhanyeni, futhi ilimaze abantu futhi ilimaze ezimeni ezibucayi.Okusho ukuthi, uma ibhethri le-asidi yomthofu selishajwe ngokweqile, lizonyusa ithuba lokuqhuma.Njengamanje, amabhethri e-lead-acid emakethe awenzanga “ukuvikela ukushajwa ngokweqile”, okwenza amabhethri e-lead-acid ekushajweni, ikakhulukazi ekupheleni kokushaja, abe yingozi kakhulu.
Okokugcina, uma isakhiwo sebhethri sonakalisiwe ngenxa yokushayisana ngengozi, amabhethri e-lead-acid abonakala ephephile kunamabhethri e-lithium.Kodwa-ke, kuleli zinga lengozi, izinto zebhethri sezivele zivezwe endaweni evulekile, futhi ukuqhuma akunakwenzeka ukukhuluma ngakho.
Kusukela ezingozini zokuphepha ezingenhla zamabhethri e-lead-asidi namabhethri e-lithium iron phosphate, kungabonakala ukuthi ingozi enkulu yokuphepha yamabhethri e-lead-acid ilele ezintweni eziwahlanganisayo.Ama-electrode amabhethri e-lead-acid ikakhulukazi enziwe ngomthofu nama-oxide awo, futhi i-electrolyte iyisisombululo se-sulfuric acid.Ukuzinza kwalezi zinto eziyisisekelo akuphezulu kakhulu.Uma kwenzeka ingozi yokuvuza noma yokuqhuma, umonakalo odaliwe uzoba phezulu kakhulu kunalowo wamabhethri e-lithium.
Ngokombono wokuphepha kwebhethri nokuklanywa kokuphindaphindeka, amabhethri e-lithium afanelekayo namabhethri e-lead-acid angaqinisekisa ngokugcwele ukuphepha kwabasebenzisi, futhi awukho umehluko osobala wokuphepha.Ingabe ibhethri ye-lithium noma ibhethri le-lead acid iphephile?Kulesi sigaba, isici sokuphepha seamabhethri e-lithiumisephezulu.
Isikhathi sokuthumela: Oct-28-2020